Precocious Puberty
Authors
INTRODUCTION
At puberty, maturation of the hypothalamic-pituitary-gonadal axis results in the profound physical, sexual, and emotional changes that transform a girl into a woman and a boy into a man. Difficult under normal circumstances, puberty out of synchrony with friends and classmates has the potential for significant emotional distress. If one accepts the mean ±2.5 SDs as encompassing the normal range, puberty is precocious if changes occur in a girl before 8 years (or possibly 7 years if a more recent study is correct) and in a boy before 9 years. Studies show a younger age of onset in African-American girls. Precocious puberty requires a careful history, physical examination, laboratory evaluation, and imaging studies to search for an underlying cause; however, in most children, especially girls, no abnormal cause is identified. Treatment is available for most cases of gonadotropin-dependent and gonadotropin-independent forms of precocious puberty.
Although children with precocious puberty undergo early physical and sexual development, their emotional and intellectual development is commensurate with their chronologic age. They and their parents require special counseling to help them adjust to these changes.
NORMAL PUBERTY
Physical Changes of Puberty
The detailed longitudinal studies of British children by Tanner and Marshall1, 2, 3 provide information about the sequence and timing of the physical and sexual changes that occur throughout puberty. For convenience, breast and pubic hair development in girls and genital and pubic hair development in boys were arbitrarily divided into five stages (Tables 1 and 2), referred to as Tanner breast, pubic hair, and genital stages.
Table 1. Tanner staging of female puberty
Stage | Breasts | Pubic hair |
1–Prepubertal | Elevation of papilla only | Any vellus over pubes no different from abdominal hair |
2 | Breast bud with elevation of breast and papilla and enlargement of areola | Slightly pigmented, downy hair along the labia |
3 | Further enlargement of breast and papilla with no separation of their contours | Darker, coarser, more curled hair over pubes |
4 | Projection of areola and papilla to form a secondary mound | Adult pubic hair that does not reach thighs (axillary hair) |
5–Adult | Mature breast, projection of papilla only as areola conforms to breast contour | Adult hair now on thighs |
Table 2. Tanner staging of male puberty
Stage | Genitalia | Pubic hair |
1–Prepubertal | Testes, penis, and scrotum same size and proportion as early childhood | Any vellus over pubes no different from abdominal hair |
2 | Early enlargement of testes >2 cm3; scrotal skin reddens and changes in texture | Slightly pigmented, downy hair at base of penis |
3 | Penis lengthens; testes enlarge 3–6 cm3; growth of scrotum | Darker, coarser, more curled, over pubes |
4 | Further penile and scrotal growth; testes 8–12 cm3 | Adult pubic hair that does not reach thighs (axillary hair) |
5–Adult | Genitalia adult in size and shape, testes 15–25 cm3 | Adult hair now on thighs |
Several factors seem to influence the time of initiation of puberty, including genetic factors, geographic location, exposure to light, and general health and nutrition. In family studies, the onset of the mother’s puberty seems more important than the father’s puberty; the daughters and sons of early-maturing mothers are early to mature.4 Children closer to the equator, children at lower altitudes, children in urban areas, and obese children start earlier than children in northern latitudes, children at higher elevation above sea level, rural children, and normal-weight children. British girls begin menarche at a mean age of 13.5 years, whereas a 1970 study showed that American girls begin menarche at a mean age of 12.8 years.5
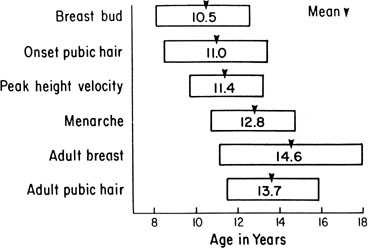
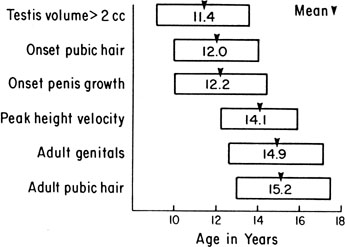
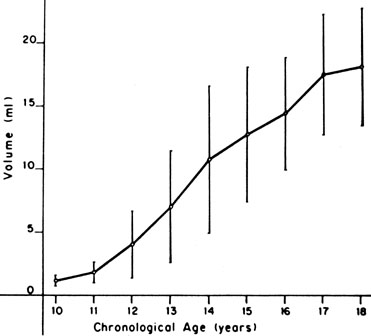
The sequence and timing of pubertal changes in girls and boys are shown in Figs. 1 and 2. Fig. 1 has been modified from the data of Marshall and Tanner2 to take into account the fact that menarche in American girls is 0.7 years earlier than in British girls. The first sign of puberty in a girl is breast budding, which occurs at a mean age of 10.5 years; the first sign of puberty in boys is testicular enlargement, which occurs a year later at a mean age of 11.5. The size of the testes can be measured accurately by comparison with a set of elliptical beads of increasing volume (the Prader orchidometer). Normal values for testicular volume are presented in Fig. 3.6 The normal range (mean ± 2.5 SD) is 8–13 years for breast budding and 9–14 years for testicular enlargement. In about 20% of children, pubic hair growth is the first sign of puberty. A cross-sectional study based on pubertal staging of more than 17,000 girls from age 3–12 years was carried out in pediatric office practices across the United States to evaluate the age of onset of puberty in African-American and white girls. This study reported that white girls had onset of pubic hair growth at a mean of 10.51 years, breast development at 9.96 years, and menarche at 12.88 years. In comparison, African-American girls had the onset of pubic hair growth at a mean of 8.78 years (range, 4.8–12.8 years), breast development at 8.87 years (range, 5–12.7 years), and menarche at 12.16 years (range, 9.7–14.6 years).7 The maximal growth rate occurs 1 year after onset of breast budding in girls at a mean age of 11.5 years and 2.5 years after testicular enlargement in boys at a mean age of 14.1 years. A criticism of this cross-sectional study is the fact that pubertal staging was rated by inspection only and not palpation. It is possible that occasional erroneous classification of a Tanner 1 girl as a Tanner 2 girl may have occurred. This cross-sectional study is supported, however, by data from the National Health Examination Surveys (NHES) from 1963 to 1970 and the National Health Examination Survey (NHANES III) from 1988 to 1994. The NHANES data indicate that 12–14% of girls have Tanner 2 breast development by age 8 years. The median age of Tanner 2 breast development is 9.5 years in the aforementioned cross-sectional study and 9.7 years in NHANES. The onset of menses is 12 years in all three studies, supporting the idea that although the onset of puberty is occurring earlier, it is not being completed earlier.8 Based on these studies, it has been proposed that the lower limit of normal onset of puberty be redefined to 7 years for white girls and 6 years for African-American girls. An organic cause for precocious puberty should be sought in white girls less than 7 years old and African-American girls less than 6 years old. No change has been made in guidelines for the assessment of precocious puberty in boys, who still require investigation for organic causes if the onset of puberty occurs at less than 9 years of age.9 Girls and boys who experience rapid progression of pubertal development, even if it occurs after the above-mentioned age cutoffs, require diagnostic studies to determine the cause of their accelerated puberty.
|
|
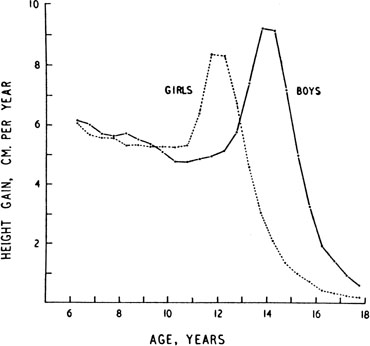
The pattern of growth velocity from birth to adult life, including the timing of the maximal pubertal growth spurt, is illustrated in Fig. 4. The maximal growth rate always precedes menarche. The mean age of menarche is 12.8 years, with a normal range of 10.3 to 15.3 years (mean ± 2.5 SD). Menarche occurs 2.3 ± 2.1 years after breast budding. Although there is not a corresponding landmark in boys, a Scandinavian study reported the average age of spermarche (as judged by appearance of spermatozoa in the urine) to be 13.4 years (range, 11.7–15.3 years).10 The age of menarche has been decreasing at a rate of about 1 year for every 25 years from 1830 to 1960. This change probably is related to improved general health and nutrition in particular. Over the last generation, this trend has leveled off.
All these figures may vary depending on the individual’s genetic and environmental background, and there is a wide variation of normal for the onset and sequence of each stage. The pubertal growth spurt coincides with increasing serum insulin-like growth factor I (IGF-I) concentrations concomitant with the rise in sex steroid levels.11 Evidence supports the conclusion that estrogens and androgens augment human pituitary growth hormone (hGH) secretion. Studies using 24-hour sampling show higher mean hGH concentrations, with more hGH peaks and higher peak amplitude.12 Children with precocious puberty and hGH deficiency still undergo a growth spurt, however, although less than normal, and manifest serum IGF-I concentrations intermediate between prepubertal children with hGH deficiency and children with sexual precocity and intact hGH secretion.13 These studies support a smaller but direct effect of sex steroids on IGF-I production.
Other important somatic changes accompany the pubertal growth spurt. In girls, total body fat increases from 16% to 23.5% of total body weight; in boys, total body fat decreases in early to mid puberty. In boys, muscle mass, as measured by radiologic studies of muscle cross-sectional area, increases fourfold; in girls, muscle mass increases twofold.
It has been proposed that when girls reach a critical weight of 47.8 kg, menarche is triggered (the so-called Frische-Revelle hypothesis).14 Other studies show that there is a large variation in total body weight at menarche, and menarche seems better correlated with the increase in total body fat to 23.5%. There are exceptions to this hypothesis, however: girls with idiopathic central precocious puberty may undergo menarche with a total body fat of 19%, children with sexual precocity secondary to hypothyroidism have a total body fat of 29%, and obese girls with no signs of puberty may have a total body fat of 27%.15 It seems more reasonable to hypothesize that central mechanisms activate the hypothalamic-pituitary axis (HPA), leading to gonadotropin stimulation of ovarian sex steroids that in turn stimulate growth to the critical weight and an increase in body fat composition to 23.5%.
The cross-sectional study cited earlier documented a trend toward initiation of puberty at earlier ages in white and African-American girls. This trend may be linked to an increase in the prevalence of obesity in young girls. Significantly higher body mass index z scores have been shown in white pubertal versus prepubertal 6–9 year-old girls. In African-American girls, a smaller difference in body mass index z score was detected, suggesting that other genetic and environmental influences may play a role in their earlier pubertal onset. The practical implications of these findings for clinicians are that white and African-American girls who are overweight may be more likely to display signs of early puberty. Physicians should consider the contribution of obesity to the incidence of early puberty when decisions regarding searching for pathologic causes or consideration of treatment of early puberty are made.16
Leptin produced by adipocytes may play a role in modulating reproduction. Leptin-deficient Ob/Ob mice are infertile, with fertility restored with leptin replacement. In rats, minimal leptin levels seem to be required to initiate puberty. Serum leptin concentrations are correlated positively with Tanner stages, and adolescent girls have higher levels than boys. Obese adolescent girls have higher serum leptin concentrations, suggesting that these girls overcome leptin resistance to achieve reproductive capacity.17
Endocrine Changes at Puberty
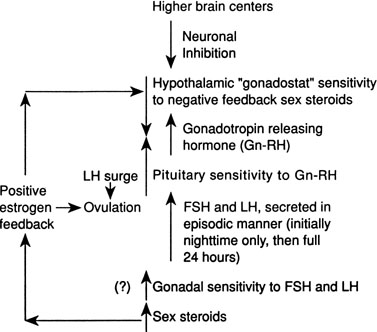
Although several pieces of the puzzle of the mechanism of onset of puberty have been put into place, the complete picture of the factors responsible for activation of the hypothalamic-pituitary-gonadal axis is not clear. During fetal life, pituitary follicle-stimulating hormone (FSH) and luteinizing hormone (LH) concentrations rise and peak near midgestation; gonadotropin levels then fall, perhaps under the inhibition of placental steroids. Early in infancy, again there is a rise in gonadotropins, perhaps resulting from loss of placental steroid inhibition, with FSH predominating in girls and LH in boys. Serum gonadotropin concentrations reach levels seen in adults (FSH, 1–31 mIU/mL in women; LH, 3–25 mIU/mL in men), peak around 2–4 weeks of life, then fall to prepubertal low levels over the next several months.18, 19 These pituitary gonadotropins stimulate temporary rises in estradiol in girls (10–60 pg/mL) and testosterone in boys (60–400 ng/dL).20, 21 Even during the quiescent prepubertal period, sensitive immunofluorometric assays show pulsatile secretion, albeit at low amplitude, of LH and FSH, with nocturnal levels higher than daytime levels.22 The prepubertal pituitary can respond to exogenous gonadotropin-releasing hormone (GnRH) stimulation, with an FSH peak predominating in girls and a slight LH peak predominating in boys.23 The prepubertal gonad also shows a rise in sex steroids in response to exogenous gonadotropin stimulation.24 Immaturity of the prepubertal pituitary-gonadal axis does not seem to prevent the onset of puberty. These studies indirectly support the hypothesis that an inhibitor from higher brain centers restrains the HPA. Although the nature of this inhibitor is unknown, opioid peptides are potential candidates. In a study of pubertal boys, administration of the opioid receptor antagonist naltrexone resulted in significantly higher mean LH concentration, LH pulse frequency, and area under LH time curve.25 Alternatively, animal studies support a neuronal-to-glial signaling pathway, perhaps via increased production of growth factors, such as transforming growth factor α, directly stimulating GnRH.26
The onset of puberty may result from diminished brain inhibition or positive glial-neuronal stimulation of GnRH secretion (Fig. 5). The maturation process that triggers this pubertal alarm clock is unknown, but it seems to be tied to normal development of the HPA during fetal life and infancy, physical growth and body composition changes, and skeletal maturation.
GnRH is secreted in a pulsatile pattern about once every 90 minutes. The frequency and amplitude of this GnRH pulse generator determine the pattern of FSH and LH secretion. During infancy, GnRH secretion is elevated followed by a long period of relative quiescence that occurs until late childhood, when pubertal maturation begins. The transition period from childhood quiescence to an adolescent pattern of GnRH secretion occurs gradually rather than abruptly. Animal models and human studies indicate that hypothalamic neurons are capable of secreting GnRH throughout childhood, and small pulses of GnRH-induced LH and FSH secretion are found in 4 year-olds.27 In girls in early puberty, FSH and LH are secreted at higher frequency and amplitude at night, and there is an equal FSH and LH response to GnRH stimulation. In mid to late puberty, FSH and LH are secreted during the day and night, with higher LH peak amplitudes, both spontaneous and in response to exogenous GnRH stimulation.23 In boys in early puberty, nocturnal LH secretion predominates; in late puberty, daytime LH secretion predominates. LH secretion, spontaneous and in response to exogenous GnRH stimulation, is greater than FSH secretion throughout puberty in boys.23
Increased pituitary gonadotropin secretion results in growth and development of the gonads and a gradual increase in sex steroid production. The normal ranges of serum gonadotropins and sex steroids through the Tanner stages are presented for girls in Table 3 and for boys in Table 4. Before the pubertal rise in gonadotropins, an increase in adrenal sex steroids, such as dehydroepiandrosterone (DHEA) and androstenedione, has been documented.28 Evidence supports separate control mechanisms regulating adrenarche and gonadarche, although some investigators have speculated that the prepubertal rise in these adrenal steroids may play a role in the activation of the hypothalamic 'gonadostat'.29 A disproportionate number of girls with premature adrenarche, resulting, for example, from late-onset congenital adrenal hyperplasia (CAH), experience an early onset of menarche.30
Table 3. Blood hormone concentrations during female puberty*
| Tanner stage | FSH† (mIU/ml) | LH† (mIU/ml) | Estradiol (pg/ml) | DHEA (ng/dL) | Androstenedione (ng/dL) | 17-OH-Progesterone (ng/dL) | Progesterone (ng/dL) |
| 1–Prepubertal | 2.16 ± 1.14 | 0.03 ± 0.03 | 1–20 | 31–345 | 8–50 | 3–82 | <10–33 |
| 2 | 3.44 ± 1.58 | 0.71 ± 1.04 | 10–24 | 150–570 | 42–100 | 11–98 | <10–55 |
| 3 | 4.88 ± 2.11 | 2.10 ± 2.33 | 7–60 | 200–600 | 80–190 | 11–155 | 10–450 |
| 4 | 6.19 ± 2.55 | 3.67 ± 2.22 | 21–85 | 200–780 | 77–225 | 18–230 | 10–130 |
| 5–Adult: Follicular | 6.63 ± 2.19 | 5.76 ± 3.46 | 3–100 | 215–850 | 82–240 | 15–70 | 15–70 |
| Luteal | 70–300 | 35–290 | 200–2500 |
To convert to International SI units, multiply:
FSH × 1 = IU/L LH × 1 = IU/L Estradiol × 3.67 = pmol/L DHEA × 34.67 = pmol/L Androstenedione × 34.92 = pmol/L 17-OH-progesterone × 30.26 = pmol/L Progesterone × 31.80 = pmol/L
* Taken from normal ranges reported by Endocrine Sciences, Tarzana, California, USA, 1990, except as noted below.
† Mean ± 1 SD taken from Neely EK, Hintz RL, Wilson DM et al: Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr 127:40, 1995.
Table 4. Blood hormone concentrations during male puberty*
| Tanner stage | FSH† (mIU/ml) | LH† (mIU/ml) | Testosterone (ng/dL) | DHT (ng/dL) | DHEA (ng/dL) | Androstenedione (ng/dL) | 17-OH-Progesterone (ng/dL) |
| 1–Prepubertal | 0.97 ± 0.59 | 0.05 ± 0.05 | <3–10 | <3 | 31–345 | 8–50 | 3–90 |
| 2 | 2.75 ± 1.84 | 1.80 ± 1.30 | 18–150 | 3–17 | 110–495 | 31–65 | 5–115 |
| 3 | 2.94 ± 1.55 | 1.86 ± 1.41 | 100–320 | 8–33 | 170–585 | 50–100 | 10–138 |
| 4 | 4.47 ± 1.88 | 2.65 ± 1.81 | 200–620 | 22–52 | 160–640 | 48–140 | 24–180 |
| 5–Adult | 4.91 ± 2.02 | 4.51 ± 1.99 | 350–970 | 24–65 | 250–900 | 65–210 | 24–175 |
To convert to International SI units, multiply:
FSH × 1 = IU/L; LH × 1 = IU/L; Testosterone × 34.67 = pmol/L; Dihydrotestosterone × 34.44 = pmol/L; DHEA × 34.67 = pmol/L; Androstenedione × 34.92 = pmol/L; 17-OH-progesterone × 30.26 = pmol/L
* Taken from normal ranges reported by Endocrine Sciences, Tarzana, CA, 1990, except as noted below.
† Mean ± 1 SD taken from Neely EK, Hintz RL, Wilson DM et al: Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr 127:40, 1995.
In mid to late puberty in girls, there is development of positive estrogen feedback on the hypothalamus.31 This feedback seems to be important in the maturation of the hypothalamic cyclic center in girls, and it is this positive estrogen feedback that stimulates the midcycle LH surge, which is important for ovulation.
CLINICAL FEATURES OF PRECOCIOUS PUBERTY
Signs of prepubertal development in a white girl before 7 years, an African-American girl before 6 years, or a boy before 9 years are beyond 2.5 SD from the mean and represent precocious puberty. Pubertal development of the same sex, seen predominantly as feminization in a girl, is termed isosexual precocious puberty; the occurrence of pubertal changes of the opposite sex, seen predominantly as virilization with hirsutism and clitoral enlargement in a girl or gynecomastia in a boy, is termed heterosexual precocious puberty.
In girls, sexual precocity usually begins with breast development, although pubic hair growth or vaginal bleeding may be the initial manifestation. Other signs of estrogen secretion include nipple enlargement and pigmentation, thickening of the labia minora, a change in the vaginal mucosa from shiny red to dull purple, and vaginal mucus production. Some emotional lability and cyclic mood swings may be present. Percentage of body fat composition increases, and a female body contour develops.
In boys, sexual precocity may begin with an increase in genital size, which may or may not include the testes, depending on the underlying cause. Pubic hair growth may be the first sign. Testes volume of greater than 2 mL indicates pubertal development. The testes progressively increase in volume through puberty (see Fig. 3). Other androgen effects include an increase in muscle bulk and strength, deepening of the voice, and eventually facial hair growth. Boys also may experience more frequent erections, penile emissions, and behavior changes, including increased libido and perhaps aggressive behavior.
Girls and boys develop apocrine gland activity, with perspiration and body odor, and sebaceous gland secretion, accompanied by oily skin and hair and sometimes acne. The secretion of estrogen in girls and androgen in boys results in increased growth velocity and accelerated skeletal maturation. These growth and skeletal changes are associated with increased serum IGF-I and IGF binding protein-3 concentrations and increased bone density; these changes are concordant with skeletal rather than chronologic age.32, 33
Complete forms of sexual precocity eventually result in the development of all secondary sex characteristics and acceleration in growth and skeletal maturation seen with normal pubertal development. These children must be investigated thoroughly to determine the underlying cause so that appropriate treatment, if indicated, can be selected. Incomplete forms of sexual precocity are less urgent. Isolated breast development in a girl before 7–8 years is termed premature thelarche. Isolated pubic or axillary hair growth before 7–8 years in a girl or 9 years in a boy is termed premature pubarche. These latter changes are usually the result of adrenal androgen secretion, so the term premature adrenarche often is used interchangeably with premature pubarche. Isolated vaginal bleeding before 8 years is termed premature menarche. Each of these incomplete forms of precocious puberty requires observation over time. If no other pubertal changes develop and growth and skeletal maturation remain normal, they are usually benign conditions.
CLASSIFICATION OF PRECOCIOUS PUBERTY
Precocious puberty is divided most easily into gonadotropin-dependent and gonadotropin-independent sexual precocity. Gonadotropin-dependent precocity is almost always pituitary-mediated and so is also termed central precocious puberty. Gonadotropin-independent precocity, usually a result of a gonadal or adrenal disorder, is also termed peripheral precocious puberty. Precocious puberty is 3–5 times more common in girls than in boys. A classification and relative occurrence of causes of sexual precocity are presented in Table 5, which is based on a summary of seven series previously reported.34, 35, 36, 37, 38, 39, 40
Table 5. Classification and relative occurrence of causes of precocious puberty*
Female (%) | Male (%) | |
Gonadotropin dependent (central) | ||
Idiopathic | 65 | 21 |
Hypothalamic hamartoma | 15 | 20 |
Other cerebral lesions | 7 | 26 |
Ectopic gonadotropins | 0.5 | 0.5 |
Primary hypothyroidism | ||
Gonadotropin independent (peripheral) | ||
Ovarian (cyst or tumor) | 5 | |
Testicular (including familial male precocious puberty) | 10 | |
McCune-Albright syndrome | 5 | 1 |
Adrenal-feminizing | 1 | 0 |
Adrenal-virilizing | 1 | 22 |
| Combined gonadotropin-independent and gonadotropin-dependent† |
* The relative occurrence of each disorder is based on a summary of seven series of precocious puberty.
† These cases are included in the gonadotropin-independent cases.
Most cases of precocious puberty are gonadotropin-dependent (87.5% in girls and 67.5% in boys). In 65% of girls, no underlying abnormality is found; this percentage has decreased from 80% in the early 1990s, resulting from the discovery of hamartomas in 15% of cases by newer imaging techniques. In contrast, two thirds of boys with central precocious puberty have an underlying central nervous system abnormality identified; pineal tumors and hamartomas are the most common causes.
Of gonadotropin-independent causes in girls, most represent autonomous ovarian lesions—cysts, granulosa-theca cell tumors, or part of McCune-Albright syndrome. In boys, the most common gonadotropin-independent cause is virilizing CAH. Because simple virilizing CAH represents the non–salt-losing form, it generally does not present in infancy. Autonomous testicular causes are increasingly recognized, the most common being familial male precocious puberty (also known as testotoxicosis or familial Leydig and germ cell hyperplasia).
In recent years there has been an increase in the use of topical testosterone cream by adult males. This has led to a number of young children becoming virilized through passive absorption of testosterone from either skin to skin contact or exposure to contaminated bed sheets, furniture, etc.41, 42
Lastly, initially autonomous gonadal or adrenal disorders may accelerate physical and skeletal maturation to the point where activation of central or pituitary mediated precocity develops. Although the mechanism of this activation is unknown, it most likely occurs with intermittent sex steroid production so that there is incomplete inhibition of pituitary gonadotropin secretion or after the underlying gonadal or adrenal disorder is treated, leading to reduced sex steroid inhibition. These children may have a combination of gonadotropin-independent and gonadotropin-dependent sexual precocity.
LABORATORY DIAGNOSIS
A careful history and physical examination are important in directing the laboratory evaluation. A search for exogenous estrogens (or androgens) should include not only questions regarding birth control pills, but also estrogen-containing cosmetics and 'health foods'. Symptoms and signs of hypothyroidism, possibly associated with galactorrhea, suggest this disorder as the cause of sexual precocity. Irregular café-au-lait spots and orthopedic problems or bone cysts on radiologic examination point toward McCune-Albright syndrome, whereas round café-au-lait spots, axillary freckling, and skin neurofibromas point toward von Recklinghausen’s disease. Neurologic and ophthalmologic abnormalities should raise suspicion of a cerebral abnormality associated with precocious puberty. A summary of the results of laboratory tests in disorders producing sexual precocity is presented in Table 6.
Table 6. Laboratory findings in disorders producing precocious puberty
Disorder | Gonadal size (examination or ultrasound) | Basal FSH and LH | Estradiol or testosterone | 24-h urinary 17-ketosteroids | LH-RH stimulation test | Head CT scan or MRI |
Idiopathic | Increased | ±, ↑ | ↑ | ↑ | Pubertal | Negative |
Cerebral | Increased | ±, ↑ | ↑ | ↑ | Pubertal | Positive |
Primary gonadal | Unilateral increase | ↓ | ↑ | ↑ | Flat | |
McCune-Albright syndrome | Increased | ↓ | ↑ | ↑ | Flat | |
Testotoxicosis | Increased | ↓ | ↑↑ | ↑ | Flat | |
Primary adrenal (e.g., CAH) | Prepubertal | ↓ | ±, ↑ | ↑↑ | Flat |
CAH, Congenital adrenal hyperplasia; ±, intermittent increase; ↑, increase; ↑ ↑, markedly increased; ↓, decreased.
Girls
If full signs of sexual precocity are present and basal gonadotropins and estradiol are in the pubertal range, gonadotropin-dependent precocious puberty is confirmed. Early in sexual precocity, basal daytime gonadotropins are often in the prepubertal range, however. With progression to Tanner stage 3 or 4, they eventually rise into the pubertal range, although this may not be apparent on a single sample because gonadotropins are secreted in an episodic fashion. In this circumstance, a GnRH stimulation test (2 μg/kg intravenously with samples for LH and FSH at 0, 15, 30, and 60 minutes) may help to separate gonadotropin-dependent from gonadotropin-independent sexual precocity. A predominant LH rise in response to GnRH is diagnostic of central precocious puberty. A predominant FSH rise is more often seen in premature thelarche, however, although it also can be seen in early precocious puberty. A flat response (<3 mIU/mL rise from baseline) is consistent with gonadotropin-independent precocious puberty. The GnRH stimulation test may not always lead to a clear separation of these two categories, however. Development of more sensitive third-generation immunochemiluminometric assays has resulted in the detection of the low basal LH and FSH levels seen in early precocious puberty. In one study, elevated basal LH levels greater than 0.1 IU/L were 94% sensitive and 88% specific in diagnosing central precocious puberty, whereas a threshold of 0.3 IU/L was 100% specific.43 Studies have shown that serum gonadotropin levels rise after subcutaneous injection of leuprolide.44, 45 In one study done in 32 children (11 males and 21 females) the rise of FSH and LH three hours after leuprolide injection correlated strongly with levels seen post GnRH stimulation. This provided evidence that FSH and LH peaks after subcutaneous leuprolide could be used in the diagnosis of precocious puberty.45 This is a useful alternative since GnRH is no longer readily available in the United States.
Also, in cases of gonadotropin-independent precocity that have evolved to a combination of gonadotropin-independent and gonadotropin-dependent sexual precocity, results may be misleading as to the original cause. Pelvic ultrasonography also may help to separate these two categories. Bilateral ovarian enlargement with multiple, small (<9 mm) follicular cysts is typical of gonadotropin-dependent precocity. Unilateral ovarian enlargement, primarily cystic in nature (>3 cm) suggests McCune-Albright syndrome or other benign cystic lesions, whereas a solid mass suggests an ovarian tumor.46, 47 Most ovarian tumors are large, averaging 12.1 cm in diameter in one series;48 most tumors this size are palpable on bimanual rectoabdominal examination.
When gonadotropin-dependent precocious puberty is confirmed, MRI of the brain with gadolinium contrast should be done to look for a hypothalamic-pituitary mass, such as a hamartoma, or other cerebral lesions. Idiopathic precocious puberty can be diagnosed only after cerebral causes have been excluded.
Attempts have been made to identify useful clinical and hormonal predictors of gonadotropin-dependent precocious puberty that identify girls at risk for an underlying central nervous system abnormality as the cause of their precocious puberty. A retrospective cohort study of 197 girls showed that 6% had an identifiable central nervous system abnormality. Of the 11 girls with an abnormality, only 3 were older than age 6 years. Overall, there was a 1:5 chance of finding a central nervous system abnormality in a girl younger than age 6 and a 1:50 chance of finding an abnormality in a girl older than age 6. The study identified the following independent predictors of central nervous system abnormalities: age of onset of puberty less than 6 years (adjusted odds ratio [AOR], 6.7), lack of pubic hair at diagnosis (AOR, 7.7) and estradiol level greater than 30 pg/mL (AOR, 4.1). A diagnostic tree was constructed that recommended brain imaging based on age less than 6 years and plasma estradiol level greater than 12 pg/mL. This diagnostic tree had 100% sensitivity and 56% specificity when validated by the study population. The authors cautioned, however, about the use of this diagnostic algorithm on other diverse populations before validation. This study provides some guidance but also illustrates the challenges in designing guidelines that provide 100% sensitivity and better specificity in the identification of central nervous system abnormalities in a 6–8 year-old girl with gonadotropin-dependent precocious puberty.49
If gonadotropin-independent sexual precocity associated with a unilateral ovarian mass is found, an elevated serum progesterone concentration suggests an ovarian luteoma. In difficult cases, a CT scan of the ovarian mass may define further cystic versus solid composition.
In the rare situation in which pelvic ultrasonography is compatible with gonadotropin-dependent precocious puberty, yet basal and GnRH-stimulated gonadotropins are suppressed, an ectopic source of hCG should be suspected. Measurement of serum hCG and other germ cell tumor markers such as alpha-fetoprotein and pregnancy-specific β1-glycoprotein should confirm this abnormality.50 These tumors are found most often in the ovary or brain.
If sexual precocity is associated with signs of virilization, one must consider CAH or virilizing adrenal or ovarian tumors. With an elevation of serum 17-hydroxyprogesterone and 24-hour urinary 17-ketosteroids, the diagnosis of 21-hydroxylase-deficient CAH is established, whereas an elevation of serum 11-deoxycortisol (compound S) and 24-hour urinary 17-ketosteroids leads to the diagnosis of 11-hydroxylase-deficient CAH. If these two serum hormones are normal, whereas 24-hour urinary 17-ketosteroids are elevated with adrenal androgens such as DHEA, androstenedione, or testosterone, one should consider an adrenal tumor or a virilizing ovarian tumor. Pelvic ultrasonography that shows bilateral prepubertal ovaries points toward an adrenal lesion. If elevated urinary 17-ketosteroids and serum androgens fail to suppress after a 7-day course of dexamethasone (0.75 mg/each 22.5 kg body weight), an autonomous source of sex steroids confirms the presence of an adenoma or carcinoma. Adrenal CT or angiography can be used to localize the tumor further.
If breast and genital development and vaginal bleeding are seen in a short child with a delayed bone age, primary hypothyroidism is the likely diagnosis. This diagnosis can be confirmed by finding a low serum thyroxine and elevated TSH concentration. Serum FSH and LH levels may be in the pubertal range, but these decrease after thyroid treatment. If galactorrhea is present, the serum prolactin concentration may be elevated.
Boys
The approach to the diagnosis of precocious puberty in boys is similar to that presented for girls except that assessment of ovarian enlargement by pelvic ultrasonography is replaced by direct testicular examination. With signs of full sexual precocity and bilateral testicular enlargement and a negative family history, gonadotropin-dependent precocious puberty is likely, whereas with a positive family history, familial male precocious puberty is likely. Serum testosterone is elevated in both conditions, but basal or GnRH-stimulated gonadotropins are elevated in the former and suppressed in the latter (the same precautions described for interpretation of these tests described for girls apply to boys). With gonadotropin-dependent precocious puberty, MRI of the brain is more likely to show a tumor or other cerebral lesion in boys. When the clinical and endocrine picture is compatible with an ectopic source of hCG, tumors are likely in the hypothalamus, mediastinum, or liver.51, 52
If one testis is much larger than the contralateral testis, and this latter testis is atrophied, this points toward a testicular tumor. In this situation, serum gonadotropins are suppressed, whereas 24-hour urinary 17-ketosteroids are elevated, and serum testosterone or androstenedione may be elevated. If both testes are prepubertal in volume, this points toward CAH or an adrenal tumor or possibly a small testicular tumor. If specific serum hormone measurements exclude CAH, CT scan of the adrenals may disclose a mass, whereas an ultrasound examination of the testes may locate a small, nonpalpable testicular tumor.
TREATMENT
Gonadotropin-Dependent
Children with precocious puberty are at risk for psychological consequences of their early sexual development and reduced adult stature from their accelerated skeletal maturation. GnRH agonists, whose continuous stimulus results in initial stimulation followed by down-regulation of the gonadotropin receptor, have proved to be relatively safe and effective treatment for children with gonadotropin-dependent precocious puberty. Six commercial GnRH agonists, differing slightly in amino acid structure and metabolic clearance and relative potency, have been used to treat central precocious puberty (Table 7).53, 54 Most are administered by daily subcutaneous injections. One drug, leuprolide, is available as a depot preparation given by the intramuscular route every 28 days or as a once every three month preparation. Treatment with GnRH agonists leads to increased gonadotropins and sex steroids for the first 7–10 days, followed by desensitization and suppression of gonadotropin secretion. This initial stimulation may result in withdrawal uterine bleeding. Individual doses must be titrated for each drug and each patient. Experience with leuprolide has shown that a starting dose between 24 and 40 μg/kg/day55 results in suppression of gonadotropin secretion and decreased sex hormone levels, preventing further progression of pubertal development. The initial dose of depot leuprolide has been the subject of much debate in recent years. In one French study, 85% of patients achieved adequate pubertal suppression on a fixed dose of 3.75 mg sc for children greater then or equal to 20 kg.56 Another study out of Stanford University suggested that a dose of 7.5mg sc every 28 days was superior to other doses in suppression as demonstrated by a depot leuprolide stimulation test. However, there was no significant difference in the final sex steroid concentrations measured on these different doses.57 The three month depot leuprolide preparation has been shown to be effective at a fixed dose of 11.25mg sc.57, 58 However, no consensus has been reached as to which dose provides the best suppression of the gonadal axis while preserving the greatest potential for target height.59 It would therefore be prudent to monitor these patients closely for pubertal progression regardless of their depot leuprolide dosage.
Side effects of GnRH agonist treatment may include local reactions such as erythema, induration, and wheal and sterile abscess formation; negative effects on growth velocity; and transient headaches, hot flushes, and withdrawal bleeding.60
Table 7. Gn-RH agonists used for treatment of central precocious puberty
Generic name (trade name) | Structure | Manufacturer | Route | Relative potency (compared with natural Gn-RH) |
Naferelin (Syneril) | [6-D-(2-naphthyl)alanine]Gn-RH acetate | Syntex | Subcutaneous, intranasal | 20–30 |
Leuprolide acetate (Lupron) | [D-Leu6des-gly-NH proethylamide9]Gn-RH | TAP | Subcutaneous, intramuscular | 20–30 |
Histrelin (Supprelin) | [imBzl-DHisb, Pro9-ethylamide]Gn-RH | Ortho | Intranasal | 140 |
Candidates for GnRH agonist therapy must be selected carefully. The tempo of pubertal progression is important when considering treatment of precocious puberty. A 1999 study looked at the initial presentation and 12-year follow-up of 20 girls with unsustained or slowly progressive puberty. All of these patients had onset of breast development or menses before age 8 years. None of the patients had a pubertal LH response to GnRH stimulation, and all had a FSH-predominant response. No patient had an elevation of estradiol above the detection limit of the assay (10 pg/mL). A 12-year follow-up questionnaire on 16 of these 20 patients showed that 70% of these girls experienced no further progression of their early puberty, whereas the remainder experienced slow progression of early puberty. The slowly progressive group was older and had a more advanced bone age compared with girls who had no progression in early puberty. Both groups achieved a final height consistent with their genetic target height and a normal average age of menarche. No increase in the incidence of anovulation was shown in this cohort. Two of these patients achieved a pregnancy. These data suggest that girls who develop on the early side of the normal age range achieve a normal height and reproductive outcome if their puberty progresses slowly despite their significantly advanced bone age and reduced height SD score for bone age. Nonprogressive or slowly progressive puberty in young girls does not warrant GnRH agonist treatment.61 In another study examining the tempo of puberty, 54 Northern Spanish girls with early onset of breast development between 8 and 9 years of age were followed to final height. The girls with normal birth weight progressed through puberty slowly with a normal timing of menarche and normal final height; however, girls of low birth weight (−1.5 SD) progressed more rapidly to menarche and ended up shorter as expressed by parental adjusted height SD. These findings suggest that a subgroup of girls with precocious puberty and low birth weight may benefit from GnRH agonist treatment.62
Long-term studies lasting to final adult height show that the best response is seen in children started on treatment before 5 years of age63 and in children whose predicted adult height is less than 155 cm.64 Another study looked at final adult height compared with predicted height in 98 children in a long-term trial of GnRH agonist treatment. In the 80 girls, final height was 159.8 ± 7.6 cm, which was greater than pretreatment predicted height (149.3 ± 9.6 cm) but still less than midparental height (163.7 ± 5.6 cm). Final height in the 18 boys was 171.1 ± 8.7 cm, which was also greater than pretreatment predicted height (156.1 ± 14.2 cm) but again less than midparental height (178.3 ± 5.2 cm). A shorter delay in the onset of treatment, longer duration of treatment, and younger chronologic and bone age at onset of treatment all lead to a greater final height outcome. This study also concluded that treatment is effective in children who start treatment after age 6 years; the average height of the girls who started treatment between 6 and 8 years was increased significantly compared with pretreatment predicted height by 6.8 ± 6.9 cm.44
Patients must be monitored during therapy to allow correct adjustment of drug dosage, including assessment of Tanner stage, growth rate, bone age advancement, and hormone production. Signs of puberty, such as breast development, genital maturation, and pubic hair growth, should fail to progress and may regress. Growth velocity should return to normal, and the rate of bone age advancement should slow to normal or less than normal. Serial pelvic ultrasonography is another modality to monitor therapy; follicular cysts should disappear, and ovarian and uterine size do not increase and may decrease.65 Vaginal bleeding or menstrual periods cease.66 Periodic acute GnRH stimulation tests may be done to ensure suppression of gonadotropin secretion, which should be measured by newer, more sensitive assays. A depot leuprolide stimulation test was found to be sufficient to assess the adequacy of suppressive treatment. Pharmacokinetic data showed that depot leuprolide functions similar to GnRH itself in causing an acute LH surge in children. Peak LH levels 10-fold higher than basal levels are sustained 30–120 minutes after depot leuprolide injection, allowing a single LH determination during this time to reflect the maximal post–depot LH concentration. Based on these data, an LH level of less than 3 mIU/mL represented adequate suppressive treatment.67 Measurement of serum estradiol in girls is usually not helpful because secretion is episodic, and current assays lack sufficient sensitivity to detect prepubertal levels; serum testosterone determinations are more useful in boys.
Several long-term studies report the effect of GnRH agonist treatment on final or near-final adult height. As described earlier, the best results are reported in children started at younger ages. In a report from the University of California at San Francisco, the mean adult height of girls started on treatment before age 5 years was 164.6 ± 9.7 cm, whereas in girls started after age 5 years the mean adult height was 157.6 ± 6.6 cm compared with a final height of 152 ± 8.6 cm in untreated girls. The treated boys averaged 166.3 ± 12.2 cm compared with 155.6 ± 7.7 cm in untreated boys. The girls as a group ended up 1 SD below their target height, whereas the boys were 1.7 SD below their target height.63
Some children experience a marked decrease in growth velocity on GnRH agonist therapy; preliminary studies suggest that the addition of growth hormone treatment improves growth velocity and predicted adult height.68 A British study reported that girls treated with GnRH agonist and hGH had larger ovaries with an increased prevalence of a polycystic appearance on ultrasound examination.69 Another study assessed 20 girls with idiopathic gonadotropin-dependent precocious puberty who had been treated with GnRH agonist (triptorelin) for 2–3 years and whose growth velocity fell below the 25th percentile for chronologic age. Ten girls received growth hormone (0.3 mg/kg/wk) in addition to GnRH agonist treatment, whereas 10 other girls comparable in age, bone age, and duration of GnRH agonist treatment who refused growth hormone treatment served as controls. Patients in the group treated with combination therapy achieved a final adult height significantly greater than pretreatment predicted adult height (160.6 ± 1.3 cm versus 152.7 ± 1.7 cm). Their height SD score for bone age significantly increased from −1.5 ± 0.2 to −0.21 ± 0.2. The GnRH-alone group did not achieve a significant gain in adult height compared with their pretreatment predicted adult height (157.1 ± 2.5 cm versus 155.5 ± 1.9 cm). Their height SD score for bone age did not change significantly. Predicted height was achieved but not significantly surpassed. No side effects such as progression of bone age or ovarian cysts were seen with combination therapy. The authors concluded that addition of growth hormone for 2–3 years to conventional GnRH agonist treatment in patients with gonadotropin-dependent precocious puberty and a decline in growth velocity may be beneficial, although the increase in final height was modest.70
Another hypothesis for the slow growth observed in girls with gonadotropin-dependent precocious puberty during GnRH agonist treatment is that their estrogen levels are suppressed below normal prepubertal values, and this causes a reduction in their growth velocity. To address this hypothesis, a pilot study was conducted in which a minidose of estrogen replacement was given to normalize growth. Thirteen girls with gonadotropin-dependent precocious puberty (3–9 years) were treated with either a combination of GnRH agonist and 8 μg of conjugated equine estrogen (group 1) or GnRH agonist alone (group 2). Results showed that group 2 patients decreased their growth velocity from 2 ± 1.4 to −1.6 ± 1.2 SD score, whereas group 1 maintained their growth velocity and their height SD score for 2 years’ duration (p < .01). No untoward acceleration of bone age or pubertal progression was seen in group 1 receiving combination therapy. The investigators concluded that low-dose estrogen replacement was safe and effective for at least 2 years in maintaining a normal prepubertal growth velocity without any side effects. The consequences of this combination therapy on final height are not yet known, however. Because most girls with gonadotropin-dependent precocious puberty on GnRH agonist therapy achieve a normal final height, it seems that the deceleration observed during therapy has no major consequences for final height in most cases. The results of this pilot study suffice to prove a concept and may be applicable only to a subgroup of girls with gonadotropin-dependent precocious puberty and short stature to prevent temporary deceleration of growth.71
When GnRH agonist treatment is stopped, studies in general show resumption of pituitary-gonadal function with menarche an average of 1.2 years later;72 a few pregnancies have been reported. Studies to date report normal bone mineral density in treated young women.73 A 2-year longitudinal follow-up study in 47 children with gonadotropin-dependent precocious puberty or early puberty assessed bone density and body composition before, during, and after cessation of GnRH agonist therapy. Bone density, peak bone mass, and body composition were not adversely affected in patients with precocious or early puberty after GnRH agonist treatment.74 GnRH agonist therapy, although evaluated primarily in children with gonadotropin-dependent precocious puberty, also was shown to be effective in precocious puberty resulting from hypothalamic hamartomas.75 GnRH agonist therapy is not effective in gonadotropin-independent forms of precocious puberty unless they produce maturation of the hypothalamic-pituitary-gonadal axis and combined precocious puberty.
Before GnRH agonist therapy, medroxyprogesterone acetate, in an oral dose of 20–40 mg/day, was used to treat idiopathic central precocious puberty. Although medroxyprogesterone acetate is successful in slowing breast and genital development and preventing menses, it is not usually successful in slowing the accelerated growth rate and skeletal maturation.76 It may be associated with signs of glucocorticoid excess.77
If a specific cerebral cause for precocious puberty is identified, treatment is aimed at curing the underlying disorder. As described previously, most hypothalamic hamartomas grow slowly and stop enlarging when the central nervous system completes its growth at 8–12 years of age. Unless these tumors cause vision impairment, hydrocephalus, or focal neurologic abnormalities, they are best followed carefully with serial MRI studies.78 When hamartomas are complicated by gelastic seizures, neurosurgical excision may be considered in cases refractory to anticonvulsant therapy. Initial results in a few children by an Australian group seem promising.79 Neurosurgical excision of hypothalamic, pituitary, optic nerve, or pineal tumors must be individualized in each patient. If these tumors are small and do not extend around or into vital brain structures, their removal may be successful. If complete surgical excision is not possible, radiotherapy or chemotherapy should be considered.
Gonadotropin-Independent
In girls with McCune-Albright syndrome, treatment with testolactone, an aromatase inhibitor that blocks conversion of androgens to estrogens, has been tried. In a report from the National Institutes of Health, 12 girls with McCune-Albright syndrome were treated for 0.5–5 years with oral testolactone, 40 mg/kg/day.80 In the seven girls who received testolactone for at least 3 years, plasma estradiol levels decreased from 168 pg/mL at the start of therapy to 42 pg/mL at 1 year, 66 pg/mL at 2 years, and 71 pg/mL at 3 years. Ovarian volumes (determined by ultrasound measurements) decreased from 4.8 to 2.1 mL. Menses decreased from an average of 8 per year before therapy to 2 per year in year 1, 3 per year in year 2, and 4 per year in year 3. Growth velocities fell, and the rate of skeletal maturation decreased. The mean predicted adult height increased from 143 cm before treatment to 147.3 cm at year 3. Although the treatment was effective in some girls, other girls exhibited an escape from the effects of treatment after 1–3 years. Treatment with a putatively more potent aromatase inhibitor, fadrozole, has been determined to be no more effective in trials of 6 months’ duration.81
In boys with familial male precocious puberty, two different drug regimens have shown partially effective initial responses. Holland and colleagues82 treated three boys with ketoconazole, an antifungal agent that interferes with adrenal and gonadal C 17,20-desmolase activity, with lesser effects on 17α-hydroxylase and 11β-hydroxylase. Ketoconazole, given in a dose of 200 mg twice a day, reduced serum testosterone levels from 256 to 22 ng/dL, growth rate fell, and the rate of skeletal maturation was slowed over 9–12 months of therapy.83 Striking improvements in behavior were noted. In a subsequent report, these subjects 'escaped' from therapy when they developed gonadotropin-dependent precocious puberty requiring the addition of GnRH agonist therapy.84 Patients on ketoconazole must be monitored for hepatotoxicity, and a case complicated by renal and hepatic failure and interstitial pneumonitis has been reported.85 Laue and coworkers86 treated nine boys with a combination of testolactone plus spironolactone, an androgen receptor blocker. When used together (testolactone, 40 mg/kg/day, and spironolactone, 2 mg/kg/day, both orally), serum testosterone concentrations remained high (around 350 ng/dL), but the growth rate decreased, and the rate of skeletal maturation returned to normal. When eight boys were treated for a longer period, 2.4–4 years, however, all exhibited a pubertal rise in gonadotropin secretion from central activation of hypothalamic GnRH secretion. These boys had a diminishing response to treatment manifested by recurrence of clinical features of puberty and an increase in the bone maturation rate. These changes required the addition of GnRH agonist treatment.87 Optimal treatment for this condition has not been determined.
If an ovarian, testicular, or adrenal tumor is identified, surgical excision is the treatment of choice. If these tumors are metastatic, other treatment modalities, such as radiotherapy or chemotherapy, may be considered. In the case of ovarian cysts, if multiple bilateral cysts are discovered on ultrasound examination, these are usually secondary to central gonadotropin secretion. If the cyst is solitary and the contralateral ovary appears immature, this may represent an autonomous cyst, such as seen with McCune-Albright syndrome. Most such cysts can be followed expectantly. Only with significant enlargement over time or with sexual precocity not responsive to medical therapy does cyst excision seem justified.
With primary hypothyroidism, thyroid replacement prevents further progression of sexual precocity. If CAH is identified, treatment with appropriate doses of glucocorticoid (and mineralocorticoid if salt-wasting is present, for example, with the 21-hydroxylase defect) also prevents further progression of pubertal development. Initial reports of a four-drug treatment protocol using an aromatase inhibitor (testolactone) and an androgen receptor blocker (flutamide) combined with glucocorticoids and mineralocorticoids show promising results.88 Long-term studies are necessary to determine the effect on virilizing features and final height. As mentioned previously, if these patients have a bone age of 10–11 years, glucocorticoid therapy may allow the onset of gonadotropin-dependent precocious puberty.89
Careful consideration must be given to the management of psychosocial problems in all children with precocious puberty. As mentioned previously, these children have intellectual, behavioral, and psychosexual maturation in keeping with their chronologic age, not their physical or pubertal age. They do not have early sexual activity. Parents, teachers, and peers may have unrealistic expectations of their intellectual and athletic abilities, and these children may even be labeled inappropriately as developmentally delayed. A careful explanation of these considerations must be given to parents. Children should be counseled that their secondary sexual characteristics are normal albeit early. If the child is bright, advancement in school may be possible with special tutoring and may prove beneficial. Children with precocious puberty may place stress on the marital or family relationship, and in these situations formal psychological counseling may be indicated.
PROGNOSIS
In children with idiopathic precocious puberty, the major long-term sequela is short stature as an adult. Without treatment, girls average 154 cm, 9.5 cm shorter than the 50th percentile, whereas boys average 164 cm, 12.5 cm shorter than the 50th percentile. Although GnRH agonist therapy stops menses if they have started and prevents further progression of pubertal development, the main therapeutic benefit is preservation of future growth potential. In an initial report from the US National Institutes of Health, 14 of 161 children had reached final adult heights, and 30 had reached 'proximate' adult heights. Of these 44 children, the girls were 157 cm, 5.2 cm above pretreatment adult height predictions but still 6.5 cm below midparental target heights. The boys were 168 cm, 6.7 cm above pretreatment adult height predictions but 8.5 cm below midparental target heights.90 As described earlier, in a report from the University of California at San Francisco, girls started on GnRH agonist therapy before 5 years of age achieved a height of 164.6 cm, whereas the boys achieved a height of 166.3 cm.63 An important question is whether normal pituitary function resumes when GnRH agonist therapy has been discontinued. A study from the National Institutes of Health reported that basal and GnRH-stimulated gonadotropin and sex steroid levels returned to pretreatment levels 3–12 months after GnRH agonist therapy was stopped, and 11 of 12 girls began to menstruate within 20 months.91
Psychometric testing shows that girls with precocious puberty have higher verbal IQ scores.92 Behavioral testing shows that most girls do not have problems; a few may show a tendency toward social difficulties related to apparent age and physical maturation, such as depression, social withdrawal, moodiness, aggression, and hyperactivity.93 Another study reported further details of the emotional and behavioral effects of having experienced precocious puberty in 19 girls who had received GnRH agonist treatment for gonadotropin-dependent precocious puberty. The results of the interview showed the patients overall were satisfied with their pediatrician’s care (95%); despite this, 53% still experienced great fear before their physician visits. During treatment, the girls experienced feelings of shame (47%) and insecurity (63%), mostly secondary to their tallness and early breast development. Also the girls felt more mature than their peers (73%), had occasional or frequent withdrawal (68%), and had fears of short final adult height (47%). In contrast, the girls tended to present themselves as socially desirable and acceptable. No decrease in physical self-esteem or discomfort was noted. An increase in internalizing feelings of depression and anxiety and total problem behavior scores compared with controls from the literature was shown. Finally, short adult height and late onset of precocious puberty were negative predictors of ultimate psychological adjustment. This study underscores the necessity to offer girls with precocious puberty psychological counseling, especially girls with a relatively late onset of puberty and short final height.94
Adolescent sexual activity tends to occur earlier, but still within the normal range of current standards.95 Most women have normal fertility, and they do not have premature menopause.96
When there is an underlying abnormality causing precocious puberty, the prognosis depends on the specific pathology. In the cerebral category, the prognosis with hypothalamic hamartomas is generally good. If other tumors of the central nervous system are completely resectable, the prognosis is good; however, this tends to be the exception rather than the rule. Craniopharyngiomas are developmental remnants rather than true neoplasms, and with partial resection and radiation therapy patients may go into remission for many years. With other conditions, such as congenital cysts, hydrocephalus, encephalitis, and neurofibromatosis, the prognosis is related to associated neurologic deficits.
With primary hypothyroidism, the prognosis is good. Children with CAH tend to be short as adults (women average 154 cm, men average 164 cm). Women tend to have problems with amenorrhea or irregular menses or infertility, although most women still achieve fertility, whereas most men have normal sperm counts. Removal of benign ovarian tumors or cysts and benign testicular or adrenal tumors carries a good prognosis, whereas malignant tumors, such as ovarian chorioepitheliomas and ovarian, testicular, and adrenal carcinomas, often have metastatic disease at the time of presentation, and the prognosis is poor. Approximately 20% of granulosa cell tumors are malignant, and the prognosis should be guarded because recurrences 25 years after removal have been reported. Approximately 25% of ovarian arrhenoblastomas are malignant.
In summary, in children with idiopathic precocious puberty or children with benign abnormalities, the prognosis is good if the children enter adult life without psychosexual scars.
REFERENCES
Tanner JM: Growth at Adolescence. 2nd ed.. Oxford, Blackwell Scientific Publishers, 1962 |
|
Marshall WA, Tanner JM: Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291, 1969 |
|
Marshall WA, Tanner JM: Variations in the pattern of pubertal changes in boys. Arch Dis Child 43:13, 1970 |
|
Garn SM, Sullivan TV: Are parent-child resemblances in growth sex-limited? Am J Dis Child 141:127, 1977 |
|
Zacharias L, Wurtman RJ, Schutzoff M: Sexual maturation in contemporary American girls. Am J Obstet Gynecol 108:833, 1970 |
|
Zachmann M, Prader A, Kind HP, et al: Testicular volume during adolescence: Cross-sectional and longitudinal studies. Helv Paediatr Acta 29:61, 1974 |
|
Herman-Giddens ME, Slora EJ, Wasserman RC, et al: Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings Network. Pediatrics 99:505, 1997 |
|
Lee PA, Kulin HE, Guo SS: Age of puberty among girls and the diagnosis of precocious puberty. Pediatrics 107:1493, 2001 |
|
Kaplowitz PB, Oberfield SE: and the Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society: Reexamination of the age limit for defining when puberty is precocious in girls in the United States: Implications for evaluation and treatment. Pediatrics 104:936, 1999 |
|
Nielsen CT, Skakkebaek ND, Richardson DW, et al: Onset of release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair and height. J Clin Endocrinol Metab 62:532, 1986 |
|
Luna AM, Wilson DM, Wibbelsman J, et al: Somatomedins in adolescence: A cross-sectional study of the effect of puberty on plasma insulin-like growth factor I and II levels. J Clin Endocrinol Metab 57:208, 1986 |
|
Ross JL, Pescovitz OH, Loriaux DL, et al: Growth hormone dynamics in children with precocious puberty. J Pediatr 110:309, 1987 |
|
Cara JF, Burstein S, Cuttler L, et al: Growth hormone deficiency impedes the rise in plasma insulin-like growth factor I levels associated with precocious puberty. J Pediatr 115:64, 1989 |
|
Frische RE, Revelle R: Height and weight at menarche and a hypothesis of menarche. Arch Dis Child 46:695, 1971 |
|
Crawford JD, Osler DC: Body composition at menarche: The Frische-Revelle hypothesis revisited. Pediatrics 56:449, 1975 |
|
Kaplowitz PB, Slora EJ, Wasserman RC, et al: Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics 108:347, 2001 |
|
Hassink SG, Sheslow DV, de Lancey E, et al: Serum leptin in children with obesity: Relationship to gender and development. Pediatrics 98:201, 1996 |
|
Kaplan SL, Grumbach MM: The ontogenesis of human foetal hormones: II. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) Acta Endocrinol 81:808, 1976 |
|
Winter JSD, Faimen C, Hobson WC, et al: Pituitary gonadal relationships in infancy: I. Patterns of serum gonadotropin concentrations from birth to four years of age in men and chimpanzee J Clin Endocrinol Metab 40:545, 1975 |
|
Winter JSD, Hughes IA, Reyes FI, et al: Pituitary-gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentration in man from birth to two years of age J Clin Endocrinol Metab 42:679, 1976 |
|
Forest MG, Cathiard AM: Pattern of plasma testosterone and 4-androstenedione in normal newborns: Evidence for testosterone activity at birth. J Clin Endocrinol Metab 41:977, 1975 |
|
Dunkel L, Alfthan H, Stenman UH, et al: Pulsatile secretion of LH and FSH in prepubertal and early pubertal boys revealed by ultrasensitive time-resolved immunofluorometric assays. Pediatr Res 27:215, 1990 |
|
Oerter KE, Uriarte M, Rose SR, et al: Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab 71:1251, 1990 |
|
Gaul C, Kasten AJ, Schally AV: Clinical experience with the use of hypothalamic releasing hormone. Recent Progr Horm Res 28:201, 1972 |
|
Mauras N, Veldhius JD, Rogol AO: Role of endogenous opiates in pubertal maturation: Opposing actions of naltrexone in prepubertal and later pubertal boys. J Clin Endocrinol Metab 62:1256, 1986 |
|
Rage F, Hill DF, Sena-Esteves M, et al: Targeting transforming growth factor alpha expression to discrete loci of the neuroendocrine brain induces female sexual precocity. Proc Natl Acad Sci U S A 94:2735, 1997 |
|
Palmert MR, Boepple PA: Variation in the timing of puberty: Clinical spectrum and genetic investigation. J Clin Endocrinol Metab 86:2364, 2001 |
|
Lee PA, Kowarski A, Migeon CJ, et al: Lack of correlation between gonadotropin and adrenal androgen levels in agonadal children. J Clin Endocrinol Metab 40:664, 1975 |
|
Sklar CA, Kaplan SL, Grumbach MM: Evidence for dissociation between adrenarche and gonadarche: Studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab 51:548, 1980 |
|
Miller D, Emans SJ, Kohane I: Follow-up study of adolescent girls with a history of premature pubarche. J Pediatr 18:301, 1996 |
|
Jaffe RN, Keye WR Jr: Estradiol augmentation of pituitary responsiveness to gonadotropin-releasing hormone in women. J Clin Endocrinol Metab 39:850, 1974 |
|
Kanety H, Karasik A, Pariente C, Kauschansky A: Insulin-like growth factor-I and IGF binding protein-3 remain high after GnRH analogue therapy in girls with central precocious puberty. Clin Endocrinol 45:7, 1996 |
|
Neely EK, Bachrach LK, Hintz RL, et al: Bone mineral density during treatment of central precocious puberty. J Pediatr 127:819, 1995 |
|
Thumdrup E: Precocious Sexual Development. Springfield, IL, Charles C Thomas, 1961 |
|
Seckel HPG: Precocious sexual development in children. Med Clin North Am 30:183, 1946 |
|
Jolly H: Sexual Precocity. Springfield, IL, Charles C Thomas, 1955 |
|
Wilkins L: The Diagnosis and Treatment of Endocrine Disorders in Childhood and Adolescence. 3rd ed.. Springfield, IL, Charles C Thomas, 1965 |
|
Sigurjonsdottir TT, Hayles AB: Precocious puberty: A report of 96 cases. Am J Dis Child 115:309, 1968 |
|
Lyon AJ, DeBruyn R, Grant DB: Isosexual precocious puberty in girls. Acta Paediatr Scand 74:950, 1985 |
|
Pescovitz OH, Comite F, Hench K, et al: The NIH experience with precocious puberty: Diagnostic subgroups and response to short-term luteinizing hormone releasing hormone analogue therapy. J Pediatr 108:47, 1986 |
|
Kunz GJ, Klein KO, Clemons RD et al: Virilization of young children after topical androgen use by their parents. Pediatrics. 2004 Jul;114(1):282-4. |
|
Brachet C, Vermeulen J, Heinrichs C: Children's virilization and the use of a testosterone gel by their fathers. Eur J Pediatr. 2005 Oct;164(10):646-7. Epub 2005 Jul 16. |
|
Verrotti A, Ferrari M, Morgese G, Chiarelli F: Premature thelarche: A long-term follow-up. Gynecol Endocrinol 10:241, 1996 |
|
Klein KO, Barnes KM, Jones JV, et al: Increased final height in precocious puberty after long-term treatment with LHRH agonists: The National Institutes of Health experience. J Clin Endocrinol Metab 86:4711, 2001 |
|
Ibanez L, Potau N, Zampolli M et al: Use of leuprolide acetate response patterns in the early diagnosis of pubertal J Clin Endocrinol Metab. 1994 Jan;78(1):30-5. |
|
Lippe BM, Sample W: Pelvic ultrasonography in pediatric and adolescent endocrine disorders. J Pediatr 92:897, 1978 |
|
Salardi S, Orsini LF, Cacciari E, et al: Pelvic ultrasonography in girls with precocious puberty, congenital adrenal hyperplasia, obesity, or hirsutism. J Pediatr 112:880, 1988 |
|
Lack EE, Perez-Atayde AR, Murthy ASK, et al: Granulosa theca cell tumors in premenarchal girls: A clinical and pathologic study of ten cases. Cancer 48:1846, 1981 |
|
Chalumeau M, Chemaitilly W, Trivin C, et al: Central precocious puberty in girls: An evidence-based diagnosis tree to predict central nervous system abnormalities. Pediatrics 109:61, 2002 |
|
Englund AT, Geffner ME, Nagel RA, et al: Pediatric germ cell and human chorionic gonadotropin-producing tumors: Clinical and laboratory features. Am J Dis Child 145:1294, 1991 |
|
Pomariede R, Finidori J, Czernichow P, et al: Germinoma in a boy with precocious puberty: Evidence of hCG secretion by the tumoral cells. Childs Brain 11:298, 1984 |
|
Navarro C, Corretser JM, Sancho A, et al: Paraneoplastic precocious puberty: Report of a new case with hepatoblastoma and review of the literature. Cancer 56:1725, 1985 |
|
Clayton RN: Gonadotropin releasing hormone: From physiology to pharmacology. Clin Endocrinol 26:361, 1987 |
|
Hardin DS, Pescovitz OH: Central precocious puberty and its treatment with long-acting Gn-RH analogs. Endocrinologist 1:163, 1991 |
|
Lee PA, Page JG: Leuprolide Study Group: Effects of leuprolide in the treatment of central precocious puberty. J Pediatr 114:321, 1989 |
|
Carel JC, Lahlou N, Guazzarotti L et al: Treatment of central precocious puberty with depot leuprorelin. FrenchLeuprorelin Trial Group. Eur J Endocrinol. 1995 Jun;132(6):699-704. |
|
Badaru A, Wilson DM, Bachrach LK et al: Sequential comparisons of one-month and three-month depot leuprolide regimens incentral precocious puberty. J Clin Endocrinol Metab. 2006 May;91(5):1862-7. Epub 2006 Jan 31. |
|
Carel JC, Lahlou N, Jaramillo O et al: Treatment of central precocious puberty by subcutaneous injections of leuprorelin J Clin Endocrinol Metab. 2002 Sep;87(9):4111-6. |
|
Foster CM: Editorial: does lupron dosage make a difference in outcome when treating childrenwith precocious puberty? J Clin Endocrinol Metab. 2006 May;91(5):1667-8. |
|
Tonini G, Lazzerini M: Side effects of GnRH analogue treatment in childhood. J Pediatr Endocrinol Metab 13(suppl 1):795, 2000 |
|
Palmert MR, Malin HV, Boepple PA: Unsustained or slowly progressive puberty in young girls: Initial presentation and long-term follow-up of 20 untreated patients. J Clin Endocrinol Metab 84:415, 1999 |
|
Ibáñez L, Ferrer A, Marcos MV, et al: Early puberty: Rapid progression and reduced final height in girls with low birth weight. Pediatrics 106:1, 2000 |
|
Paul D, Conte FA, Grumbach MM, Kaplan SL: Long-term effect of gonadotropin-releasing hormone agonist therapy on final and near-final height in 26 children with true precocious puberty treated at a median age of less than 5 years. J Clin Endocrinol Metab 80:546, 1995 |
|
Brauner R, Adan L, Malandry F, Zantleifer D: Adult height in girls with idiopathic true precocious puberty. J Clin Endocrinol Metab 79:415, 1994 |
|
Ambrosino MM, Hernanz-Schulman M, Genieser NB, et al: Monitoring of girls undergoing medical therapy for isosexual precocious puberty. J Ultrasound Med 13:501, 1994 |
|
Boepple PA, Mansfield MJ, Wierman ME, et al: Use of a potent, long-acting agonist of gonadotropin-releasing hormone in the treatment of precocious puberty. Endocr Rev 7:24, 1986 |
|
Bhatia S, Neely EK, Wilson DM: Serum luteinizing hormone rises within minutes after depot leuprolide injection: Implications for monitoring therapy. Pediatrics 109:1, 2002 |
|
Pasquino AM, Municchi G, Pucarelli T, et al: Combined treatment with gonadotropin-releasing hormone analog and growth hormone in central precocious puberty. J Clin Endocrinol Metab 81:948, 1996 |
|
Bridges NA, Cooke A, Healy MJ, et al: Ovaries in sexual precocity. Clin Endocrinol 42:135, 1995 |
|
Pucarelli I, Segni M, Ortore M, et al: Combined therapy with GnRH analog plus growth hormone in central precocious puberty. J Pediatr Endocrinol Metab 13(suppl 1):811, 2000 |
|
Lampit M, Golander A, Guttmann H, Hochberg Z: Estrogen mini-dose replacement during GnRH agonist therapy in central precocious puberty: A pilot study. J Clin Endocrinol Metab 87:687, 2002 |
|
Boepple PA: Final height and reproductive function in central precocious puberty (CPP) following long-term pituitary-gonadal suppression induced by GnRH agonists (GnRH). 79th Annual Endocrine Society Program and Abstracts 42:1997 |
|
Saggese G: Evidence of normal peak bone mass after gonadotropin-releasing hormone analogue therapy in central precocious puberty (abstract). Horm Res 48:119, 1997 |
|
Van der Sluis IM, Boot AM, Krenning EP, et al: Longitudinal follow-up of bone density and body composition in children with precocious or early puberty before, during and after cessation of GnRH agonist therapy. J Clin Endocrinol Metab 87:506, 2002 |
|
Mahachoklertwattana P, Kaplan SL, Grumbach MM: The luteinizing hormone-releasing hormone-secreting hypothalamic hamartoma is a congenital malformation: Natural history. J Clin Endocrinol Metab 77:118, 1993 |
|
Sadeghi-Nejad A, Kaplan SL, Grumbach MM: The effect of medroxyprogesterone acetate on adrenocortical function in children with precocious puberty. J Pediatr 78:616, 1971 |
|
Richman RA, Underwood LE, French FS, et al: Adverse effects of large doses of medroxyprogesterone (MPA) in idiopathic isosexual precocity. J Pediatr 79:963, 1971 |
|
Turjman F, Xavier JL, Froment JC, et al: Late MR follow-up of hypothalamic hamartomas. Child Nerv Syst 12:63, 1996 |
|
Rosenfeld JV, Harvey AS, Wrennall J, et al: Transcallosal resection of hypothalamic hamartomas, with control of seizures, in children with gelastic epilepsy. Neurosurgery 48:108, 2001 |
|
Feuillan PP, Jones J, Cutler GB Jr: Long-term testolactone therapy for precocious puberty in girls with the McCune-Albright syndrome. J Clin Endocrinol Metab 77:647, 1993 |
|
Nunez SB, Feuillan PP, Jones J, Cutler GB Jr: Treatment of precocious puberty in MAS with the aromatase inhibitor fadrazole (abstract). Horm Res 48:124, 1997 |
|
Holland FJ, Fishman L, Bailey JD, et al: Ketoconazole in the management of precocious puberty not responsive to LHRH-analogue therapy. N Engl J Med 312:1023, 1985 |
|
Engelhardt D, Weber MM, Miksch T, et al: The influence of ketoconazole on human adrenal steroidogenesis: Incubation studies with tissue slices. Clin Endocrinol 35:163, 1991 |
|
Holland FJ, Kirsch SE, Selby R: Gonadotropin-independent precocious puberty (“testotoxicosis”): Influence of maturational status on response to ketoconazole. J Clin Endocrinol Metab 64:328, 1987 |
|
Babovic-Vuksanovic D, Donaldson MD, Gibson NA, Wallace AM: Hazards of ketoconazole therapy in testotoxicosis. Acta Paediatr 83:994, 1994 |
|
Laue L, Kenigsberg D, Pescovitz OH, et al: Treatment of familial male precocious puberty with spironolactone and testolactone. N Engl J Med 320:496, 1989 |
|
Laue L, Jones J, Barnes KM, Cutler GB Jr: Treatment of familial male precocious puberty with spironolactone, testolactone, and deslorelin. J Clin Endocrinol Metab 76:151, 1993 |
|
Merke DP, Jones J, Barnes KM, Cutler GB Jr: Two-year experience with flutamide, testolactone, and reduced hydrocortisone dose in the treatment of congenital adrenal hyperplasia (abstract). Horm Res 48:6, 1997 |
|
Pescovitz OH, Comite F, Cassorla F, et al: True precocious puberty complicating congenital adrenal hyperplasia: Treatment with a luteinizing hormone-releasing hormone analog. J Clin Endocrinol Metab 58:857, 1984 |
|
Oreter KE, Manasco P, Barnes KM, et al: Adult height in precocious puberty after long-term treatment with deslorelin. J Clin Endocrinol Metab 73:1235, 1991 |
|
Manasco PK, Pescovitz OH, Feuillan PP, et al: Resumption of puberty after long-term luteinizing hormone-releasing hormone agonist treatment of central precocious puberty. J Clin Endocrinol Metab 67:368, 1988 |
|
Galatzer A, Beth-Halachmi N, Kauli R, et al: Intellectual function of girls with precocious puberty. Pediatrics 74:246, 1984 |
|
Sonis WA, Comite F, Blue J, et al: Behavior problems and social competence in girls with true precocious puberty. J Pediatr 106:156, 1985 |
|
Baumann DA, Landolt MA, Wetterwald R, et al: Psychological evaluation of young women after medical treatment for central precocious puberty. Horm Res 56:45, 2001 |
|
Ehrhardt AA, Meyer-Bahlburg HF: Psychosocial aspects of precocious puberty (abstract). Horm Res 41:30, 1994 |
|
Murram D, Dewhurst J, Grant DB: Precocious puberty: A follow-up study. Arch Dis Child 59:77, 1984 |